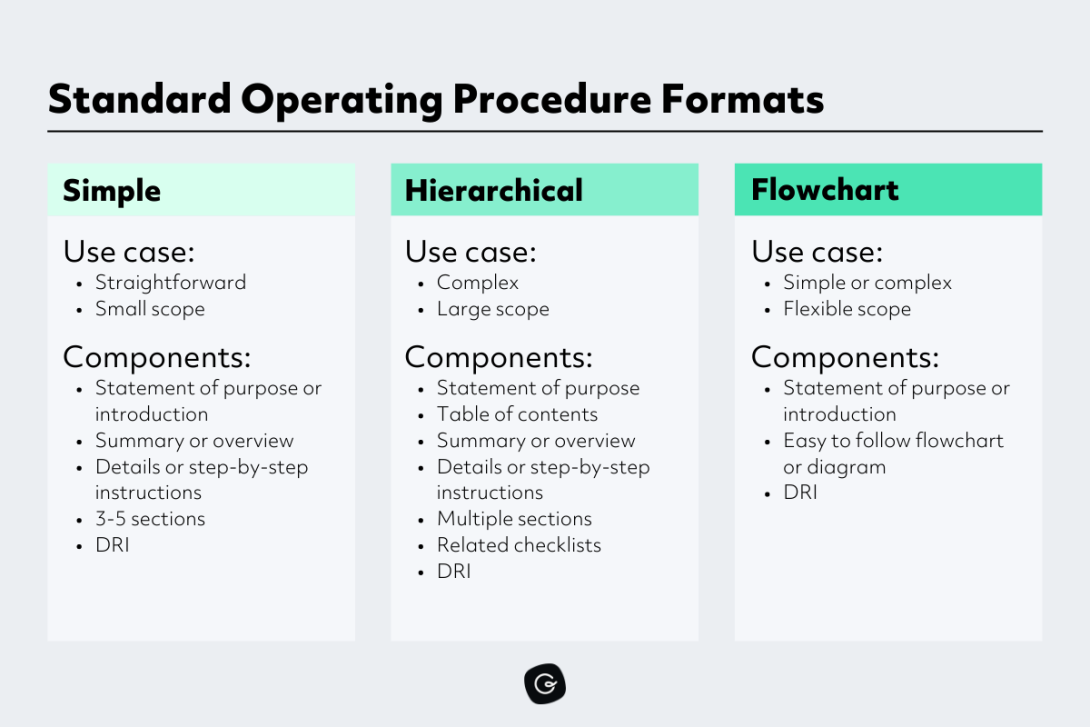
Standard Operating Procedures (SOPs) serve as essential documents within various industries and organizations, providing a structured guide for carrying out specific tasks, processes, or activities. They play a crucial role in ensuring consistency, efficiency, and quality in operations.
You May like to read –
- Technical Writing for Quality Management System (QMS)
- Risk Management in Quality Compliance | Everything To Know
- 10 Key Elements of GLP Compliance for Safe and Reliable Data
- Quality Regulatory Compliance Guide for Businesses
- GLP vs. GMP vs. GCP: Understanding the Differences
This article delves into the significance of SOP documentation, its structure, key components, and best practices for creating effective SOPs.
What is an SOP?

A Standard Operating Procedure (SOP) is a comprehensive document that outlines step-by-step instructions for performing a specific task, process, or activity. It serves as a reference guide for employees, ensuring that tasks are carried out uniformly, regardless of who is performing them. SOPs can range from simple tasks like data entry to complex processes such as manufacturing, clinical trials, or emergency response.
Read More at https://www.gxpcellators.com/sop-documentation-best-practices/
